FDA/CDC Eye Drop Recall
Eye drop manufacturers have found themselves at the center of a complex and contentious issue regarding the outbreak of eye infections in the USA. In January 2023, the Centers for Disease Control and Prevention (CDC) raised concerns about a potential link between eye drops and a surge in eye infections. These concerns stemmed from a series of infections caused by the bacterium Pseudomonas aeruginosa, which is known for its resistance to many antibiotics and its ability to cause severe health issues, particularly in hospital and healthcare settings.
The Contamination and the Outbreak
The contamination issue came to light when the Centers for Disease Control and Prevention (CDC) on January 31, 2023 began investigating a multistate cluster of Pseudomonas aeruginosa infections. The investigation focused on determining whether there was a link between the infections and various eye drop products.
The Investigation at a Long-Term Care Center
A critical part of the investigation occurred at a long-term care center in Connecticut, where CDC investigators examined an outbreak involving two dozen cases. The team noted evidence of bacterial spread among the residents, and while they suspected eyedrops as a potential source of the infections, the center's records were not detailed enough to determine the exact type of eye drops that may have been used.
Concerns and Recall
FDA asked manufacturers to voluntarily submit and recall their products. The following manufacturers acted as good corporate citizens and immediately voluntarily recalled their products without any proof that their products were contaminated.
The following companies voluntarily recalled their products.
On February 2, 2023:
- EzriCare Artificial Tears Lubricant Eye Drops (Global Pharma Healthcare Private LTD.)
- Delsam Pharma Artificial Tears
On February 24, 2023:
- Delsam Pharma Artificial Eye Ointment
All other manufacturers refused to voluntarily recall any of their eye drop products.
As investigations were conducted the FDA felt it was necessary to issue mandatory recalls to the following eye drop brands/products.
On March 1, 2023:
- Apotex Brimonidine Tartrate Ophthalmic Solution
On March 3, 2023:
- Pharmedica Purely Soothing MSM Drops
On March 20, 2023:
- Teva Pharmaceuticals Clear Eyes Once Daily
- Eye Allergy Itch Relief
As evidence from tests were gathered, the FDA issued formal warning letters to the following eye drop brands/products again asking them to issue a voluntary recall:
- CVS Health Lubricant Eye Drops
- CVS Health Multi-Action relief Drops
- Rugby Lubricating Tears Eye Drops
- Rugby Polyvinyl Alcohol 1.4% Lubricating Eye Drops
- Leader Dry Eye Relief
- Rite Aid Gentle Lubricant Gel Eye Drops
- Target Up&Up Extreme Relief Dry Eye
- Velocity Pharma LLC Lubricant Eye Drop
- Walmart Equate Hydration PF Lubricant Eye Drops
By the summer of 2023, the FDA had completed enough testing and had been able to narrow down to where they believe the problems originated. As such on August 30, 2023, the FDA ordered recall of 28 brands of eye drops that refused or failed to voluntarily recall their products:
- CVS Health Lubricant Eye Drops 15 ml (single pack)
- CVS Health Lubricant Eye Drops 15 ml (twin pack)
- CVS HealthLubricant Gel Drops 15 ml (single pack)
- CVS HealthLubricant Gel Drops 15 ml (twin pack)
- CVS Health Multi-Action Relief Drops 15 ml
- CVS Health Lubricant Gel drops 10 ml
- CVS Health Lubricant Eye Drops 10 ml (single pack)
- CVS Health Lubricant Eye Drops 10 ml (twin pack)
- CVS Health Mild Moderate Lubricating Eye Drops 15 ml (single pack)
- Leader (Cardinal Health) Eye Irritation Relief 0.5 FL OZ (15 ml)
- Leader (Cardinal Health) Dry Eye Relief 0.5 FL OZ (15 ml)
- Leader (Cardinal Health) Lubricant Eye Drops 0.5 FL OZ (15 ml) (single)
- Leader (Cardinal Health) Lubricant Eye Drops 0.5 FL OZ (15 ml) (twin pack)
- Leader (Cardinal Health) Dry Eye Relief 0.33 FL OZ (10 ml)
- Leader (Cardinal Health) Lubricant Eye Drops 0.33 FL OZ (10 ml)
- Rugby (Harvard Drug Group) Lubricating Eye Drops 0.5 oz (15 ml)
- Rugby (Harvard Drug Group) Lubricating Tears Eye Drops 0.5 oz (15 ml)
- Rite Aid Lubricant Eye Drops 15 ml (twin pack)
- Rite Aid Lubricant Eye Drops 10 ml (twin pack)
- Rite Aid Gentle Lubricant Gel Eye Drops 15 ml
- Rite Aid Lubricant Gel Drops 15 ml
- Rite Aid Lubricating Gel Drops 10 ml
- Rite Aid Multi-Action Relief Drops 15 ml
- Target Up&Up Dry Eye Relief 15 ml (twin pack)
- Target Up&Up High Performance Lubricant Eye Drops 15 ml (single pack)
- Target Up&Up High Performance Lubricant Eye Drops 15 ml (twin pack)
- Velocity Pharma LLC Lubricant Eye Drop 10 ml (triple pack)
- Walmart Equate Hydration PF Lubricant Eye Drop 10 mL
Testing and Findings
The outbreak strain belongs to sequence type (ST) 1203 and carries resistance genes blaVIM-80 and blaGES-9, which confer resistance to carbapenems. According to publicly available genome data, carbapenem resistant P. aeruginosa (CRPA) carrying both VIM-80 and GES-9 has not been previously identified (CDC, 2023).
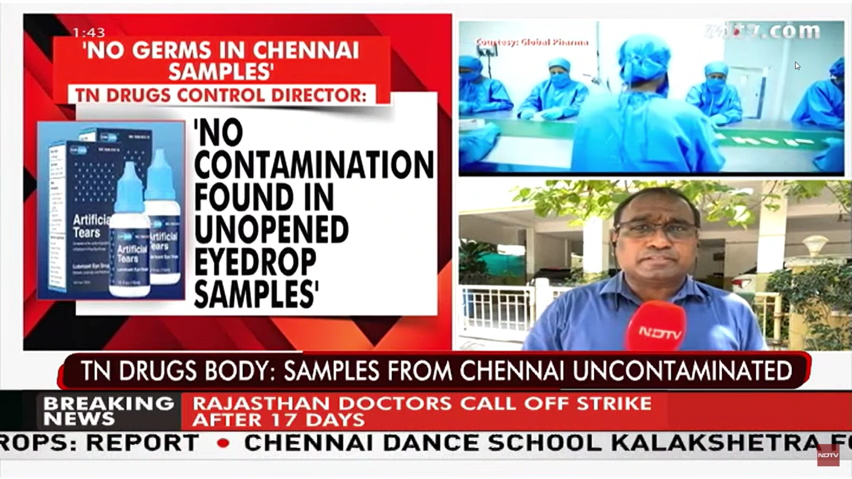
Any testing conducted by the FDA on unopened bottles of EzriCare Artificial Tears produced by Global Pharma was unable to identify any of the outbreak strain.
The VIM-GES-CRPA outbreak cluster is at least 32 SNPs distant from Indian contextual isolates, this clearly indicates that the contaminants could not have evolved from the isolate components used by Global Pharma. There is no genomic evidence substantiating the origin of VIM-GES-CRPA from Global Pharma’s facilities.
The control samples collected by the Indian drug regulator from the manufacturing plant near Chennai, India showed no contaminants. These control samples, part of the company's quality control processes, are from the same batches that were exported. Similarly, the US FDA collected samples in India, which were tested at an approved laboratory in Bengaluru, also showing no contamination.
A joint investigation conducted in February by India's apex drug regulator and the state drug controller revealed that Global Pharma adhered to stringent quality control measures. This included biannual "media fill validation" tests, used to check for microbial contamination by exposing growth medium to manufacturing surfaces, and yearly stability studies to ensure products meet stipulated specifications over time.
False Accusations/Reactions
As a result of the severity and initial reaction to this issue, numerous false assumptions and accusations were published.
Prior to reviewing or seeing the test results, numerous media outlets posted articles that implied or assumed that Global Pharma products were responsible for the adverse health effects that were experienced by users of various eye drops.
The manufacturers, specifically Global Pharma, who acted responsibly and quickly recalled their products without any evidence that their products contained any contaminants were assumed by many in the media to be guilty and negligent, whereas the manufacturers and retailers who refused to voluntarily recall any of their products were able to avoid accepting any responsibility.
These false accusations/assumptions manifested into class action lawsuits, negative publicity and significant legal issues for the people who operated good companies who were trying to do the right thing by complying with the FDA’s requests.
As an example, Global Pharma Healthcare Private LTD. was the recipient/victim of numerous false accusations which resulted in legal claims/lawsuits only to be discontinued when it became obvious that any contamination could not have possibly occurred in the Global Pharma Healthcare Private LTD. facility.
Summary
Through scientific evidence the contaminant that has caused the issues experienced by a number of users of eye drops in the United States, is a rare and extensively drug-resistant Pseudomonas aeruginosa (CRPA), carrying both VIM-80 and GES-9 . It belongs to sequence type (ST) 1203 and contains resistance genes blaVIM-80 and blaGES-9.
Through testing and findings it was proved that the VIM-GES-CRP, an outbreak cluster was at least 32 SNPs distant from Indian contextual isolates used by Global Pharma in the production of the EzriCare product. As such it is scientifically impossible that this contamination could have originated in the EzriCare products from the manufacturing unit.